Gage Tracking Software for Manufacturing: ISO 17025 Compliance Made Simple
Unai Simmons
Calibration Technician
5

Meeting ISO/IEC 17025:2017 calibration requirements doesn't have to drain your resources. Modern gage tracking software streamlines compliance, automates documentation, and eliminates the manual spreadsheets that create audit nightmares. Here's how manufacturing facilities are achieving bulletproof ISO 17025 compliance while actually reducing administrative workload.
Why ISO 17025 Compliance is Critical for Manufacturing
ISO/IEC 17025:2017 is the international standard for testing and calibration laboratories, establishing requirements for competence, impartiality, and consistent operation. For manufacturing facilities, compliance isn't optional—it's essential for:
Customer Requirements: Major OEMs and defence contractors mandate ISO 17025-compliant calibration
Regulatory Compliance: FDA, FAA, and other regulators expect traceable calibration systems
Quality Assurance: Accurate measurements prevent costly defects and recalls
Competitive Advantage: ISO 17025 certification differentiates you from competitors
Risk Mitigation: Proper calibration management protects against liability claims
The challenge? ISO 17025 requires meticulous documentation, traceability, and process control that overwhelm manual tracking systems. That's where Gaugify.io gage tracking software transforms compliance from a burden into a streamlined, automated process.
The ISO 17025 Compliance Challenge in Manufacturing
Common Pain Points Without Proper Gage Tracking Software:
1. Documentation Overload Manufacturing quality managers spend 10-15 hours per week maintaining calibration records manually. Excel spreadsheets become unwieldy, email reminders get missed, and paper certificates disappear in filing cabinets.
2. Traceability Gaps ISO 17025 Clause 6.5 requires unbroken traceability to national or international standards. Tracking this across hundreds or thousands of instruments without dedicated software creates gaps that auditors flag immediately.
3. Deadline Management Failures Missing a single calibration deadline can invalidate weeks of production data. Manual calendar systems simply can't scale to handle complex multi-location calibration schedules.
4. Audit Preparation Stress When auditors arrive, scrambling to compile compliance evidence from multiple sources reveals system weaknesses. Most facilities spend 40+ hours preparing for ISO 17025 audits.
5. Measurement Uncertainty Documentation Clause 7.6 requires calculating and documenting measurement uncertainty—a complex task that's nearly impossible to manage consistently without software support.
How Gaugify.io Gage Tracking Software Solves ISO 17025 Compliance
Gaugify.io was purpose-built to address every ISO 17025 requirement while remaining remarkably easy to use. Here's how it transforms compliance:
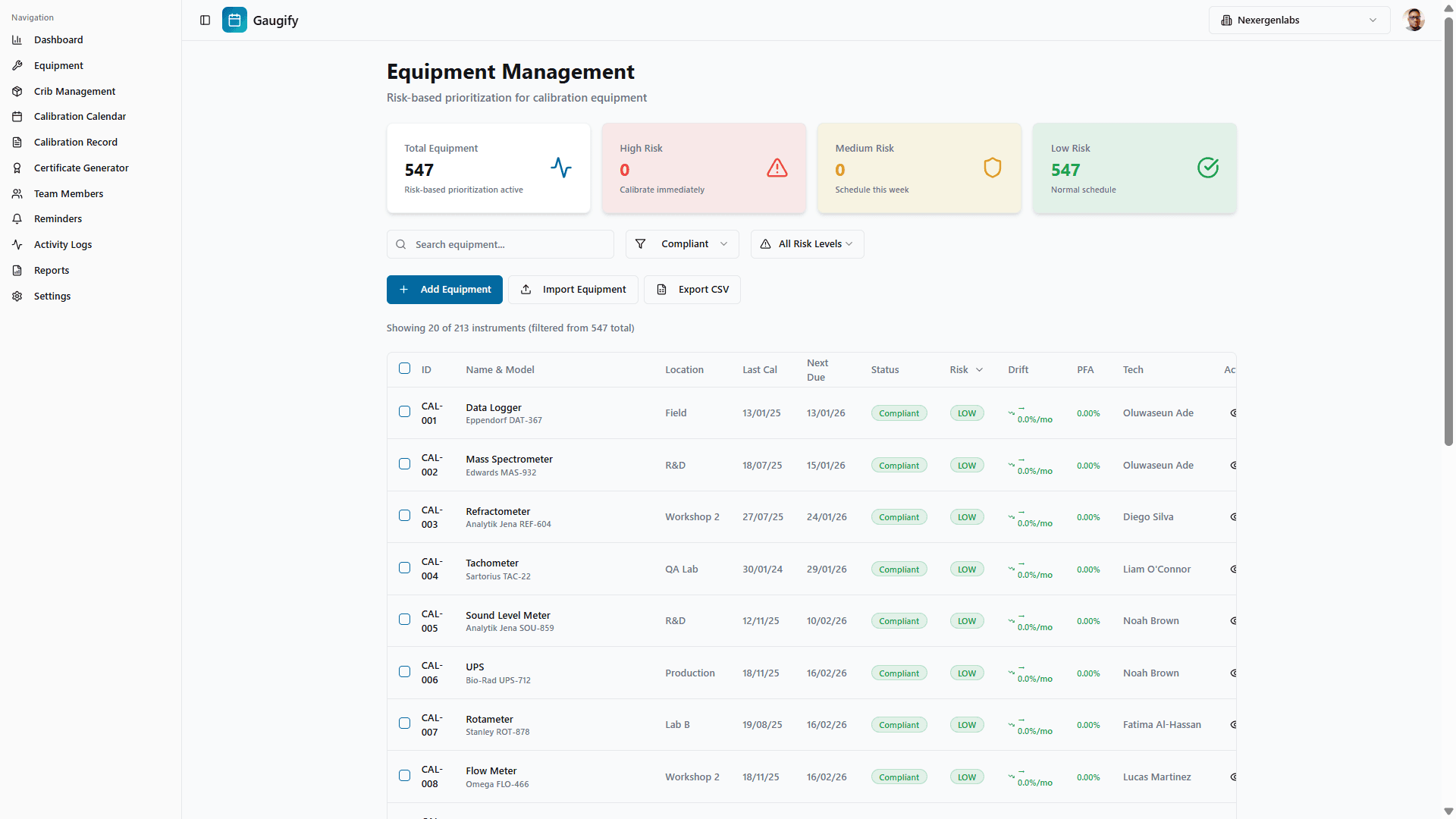
1. Automated Calibration Scheduling (Clause 6.4.13)
ISO 17025 Requirement: Equipment must be calibrated before use, at specified intervals, or when accuracy is suspect.
Gaugify.io Solution:
Intelligent scheduling engine tracks calibration due dates automatically
Customizable reminder sequences (30, 14, 7, 1 day warnings)
Escalation workflows notify supervisors of overdue items
Automatic out-of-service flagging for expired instruments
2. Complete Traceability Chain (Clause 6.5)
ISO 17025 Requirement: Metrological traceability to SI units through an unbroken chain of calibrations.
Gaugify.io Solution:
Digital certificate storage with instant retrieval
Automatic linking of calibration standards to end instruments
Traceability chain visualization showing path to national standards
NIST-traceable vendor verification and approval workflows
[PLACEHOLDER: Screenshot showing traceability chain visualization from NIST through calibration lab to shop floor instrument]
3. Calibration Status Tracking (Clause 6.4.13)
ISO 17025 Requirement: Clear identification of calibration status on all equipment.
Gaugify.io Solution:
QR code/barcode label generation with calibration status
Color-coded visual indicators (green = current, yellow = due soon, red = overdue)
Mobile app scanning for instant status verification
Automatic "Do Not Use" tagging for out-of-calibration instruments
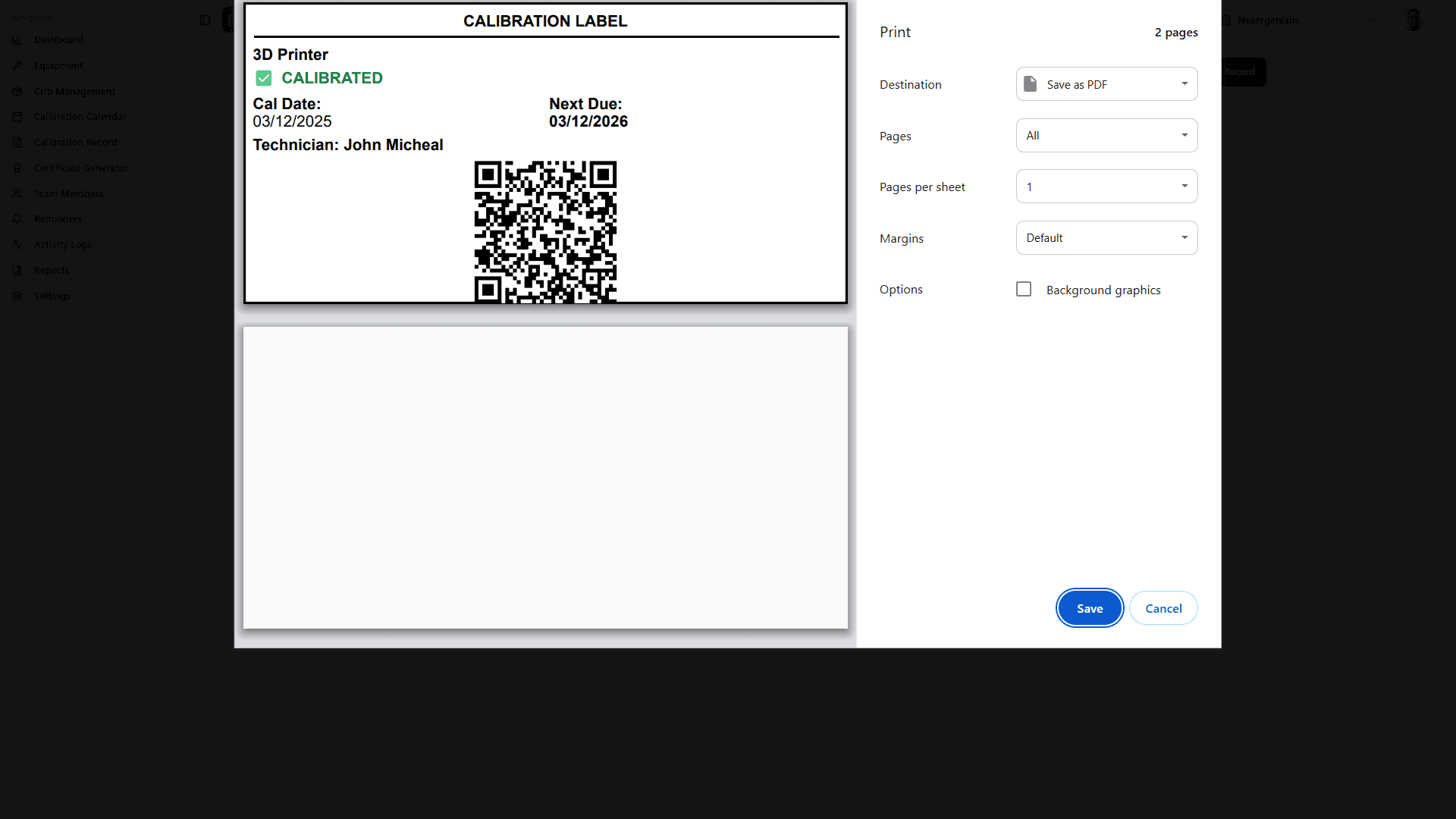
4. Measurement Uncertainty Management (Clause 7.6)
ISO 17025 Requirement: Calculate measurement uncertainty for calibrations and testing.
Gaugify.io Solution:
Built-in uncertainty calculators following GUM methodology
Automated uncertainty propagation through calibration chains
Template-based uncertainty budgets for common instrument types
Historical uncertainty trending and analysis
5. Records and Documentation (Clause 7.5)
ISO 17025 Requirement: Maintain records with sufficient detail to allow repeat calibrations.
Gaugify.io Solution:
Unlimited digital storage for calibration certificates
Complete audit trail of all calibration activities
Automatic archiving meeting ISO 17025 retention requirements
Customizable calibration procedure templates
As-found and as-left data capture with photographic evidence support
Step-by-Step: Implementing Gaugify.io for ISO 17025 Compliance
Phase 1: System Setup (Week 1)
Step 1: Inventory Your Calibration Program Before implementing any gage tracking software, document your current state:
List all measurement and test equipment requiring calibration
Identify calibration intervals for each instrument type
Document existing calibration procedures
Map your traceability requirements
Review current non-conformance and corrective action processes
Step 2: Configure Gaugify.io Settings
Step 3: Import Instrument Database Gaugify.io accepts instrument data via:
CSV import templates (provided)
Manual entry through intuitive forms
Barcode scanning for existing tagged equipment
API integration from CMMS or ERP systems
Pro Tip: Start with critical measurement equipment first. Gaugify.io's flexible structure allows you to expand gradually rather than requiring complete setup before going live.
Phase 2: Data Population (Week 2)
Step 4: Enter Calibration Standards Document your reference standards hierarchy:
Primary standards (sent to NIST or accredited labs)
Working standards (calibrated against primary standards)
Check standards for verification
For each standard in Gaugify.io, record:
Manufacturer, model, serial number
Calibration procedure reference
Acceptance criteria
Calibration interval
Assigned uncertainty
Step 5: Upload Historical Certificates Scan and upload existing calibration certificates to establish baseline traceability. Gaugify.io's OCR capabilities can extract key data from PDF certificates automatically.
Step 6: Define Calibration Procedures Create or import Standard Operating Procedures (SOPs) for each instrument type:
Equipment required
Environmental conditions
Step-by-step instructions
Acceptance criteria
Uncertainty calculations
Phase 3: Process Integration (Week 3)
Step 7: Implement Barcode/QR Code System Generate calibration labels for all instruments:
Each label includes:
Unique instrument ID
QR code linking to digital record
Calibration due date
"Scan to Update" instructions for technicians
Step 8: Train Your Team Gaugify.io's intuitive interface minimizes training requirements:
Administrators: 2-3 hours (system configuration, reporting)
Calibration Technicians: 1 hour (mobile app, data entry)
General Users: 15 minutes (status checking, requesting calibrations)
Step 9: Configure Mobile Access Deploy Gaugify.io mobile apps (iOS/Android) to calibration technicians:
Field calibration data entry
Certificate photo capture
Real-time status updates
QR code scanning for check-in/check-out
Phase 4: Go-Live and Optimization (Week 4+)
Step 10: Parallel Operation Period Run Gaugify.io alongside your existing system for 2-4 weeks:
Verify all instruments are tracked correctly
Confirm notifications are reaching appropriate personnel
Validate report accuracy
Collect user feedback
Step 11: Full Cutover Once validated, make Gaugify.io your system of record. Export final data from legacy systems for archival purposes.
Step 12: Continuous Improvement Leverage Gaugify.io's analytics to optimize your calibration program:
Identify instruments with high failure rates
Adjust calibration intervals based on stability data
Track calibration costs by department or instrument type
Monitor technician productivity and workload balance
